April 4, 2023
CSB project students present their year’s work in research
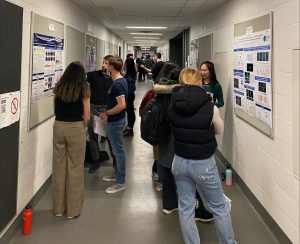 A year of research by CSB project students culminated in a poster session on March 31st, 2023. The students used the posters to…
A year of research by CSB project students culminated in a poster session on March 31st, 2023. The students used the posters to…
December 20, 2022
Congratulations to CSB’s Graduate Student Award Recipients!
Congratulations to our Graduate Students who earned recognition for their accomplishments at our Graduate Student Awards on December 19th, 2022! Valerie Anderson Graduate Fellowship Awarded for academic merit to an outstanding student in any subdiscipline of plant biology. Recipient: Saad Hussain…
January 14, 2021
Collaboration is key at CSB’s aquatic research facility
 Amanda Miles’ PhD studies in Prof…
Amanda Miles’ PhD studies in Prof…
May 16, 2019
Congratulations to Prof. Ashley Bruce and Prof. Alan Moses on their promotion to Full Professor
It is our pleasure to announce that Ashley Bruce and Alan Moses have been promoted to Full Professor in the Department of Cell and Systems Biology as of July 1, 2019.

