The Christine Hone-Buske award is given each year to a CSB graduate student who has produced an outstanding paper in their field. This year, the award went to PhD candidate Julia Gauberg for her publication “Conserved biophysical features of the CaV2 presynaptic Ca2+ channel homologue from the early-diverging animal Trichoplax adhaerens“.
Gauberg’s paper revealed insights into how the nervous system evolved by studying a wide range of animals, from sponges to snails to zebrafish, but with particular attention to the millimetre size seawater animal, Trichoplax. Trichoplax has no nerves or muscles, but can exhibit coordinated movement in response to stimuli like food or gravity.
Even though Trichoplax diverged long ago from the common ancestor with humans, Gauberg’s multi-species analysis identified calcium channels in Trichoplax called CaV2 that are very similar to the “presynaptic” calcium channels expressed in human nerve cells. Presynaptic nerve cells pass a signal across a small gap (a synapse) to a postsynaptic receiver cell. This signal is often initiated by a rush of calcium ions into the presynaptic cell through a presynaptic CaV2 channel.
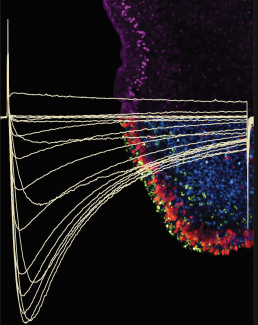
To test the activity of Trichoplax CaV2 (TCaV2), Gauberg transferred the Trichoplax gene into a human cell line. Gauberg can monitor the electrical currents across the human cell surface on specialized electrophysiology equipment and detect how currents change with an influx of calcium. She found that TCaV2 conducts high voltage–activated calcium ion currents in a manner similar to the human channel. So despite millions of years of evolution, CaV2 has retained many of its “core” properties.
For TCaV2 to truly act like a presynaptic channel, it must be present in the right location in the animal. Gauberg looked at the location of TCaV2 and saw it around the periphery of Trichoplax, in mucocytes and in a few other cell types. In addition to secreting mucus, these mucocytes release signals similar to human neuropeptides found at synapses, so this localization strongly supports the idea that TCaV2 is involved in secreting signaling molecules from this cell.
In nerve cells, once the presynaptic signal is released, it is received by the postsynaptic cell, triggering an electrical potential to propagate the signal. Gauberg’s future work will look at ion channels in Trichoplax that are most similar to the postsynaptic channel in humans to see if this aspect of cell-to-cell signaling is also preserved.
With its complex coordinated movement, Trichoplax is a unique intermediate on the path to the evolution of the nervous system and cell-to-cell signaling complexity. As the nervous system evolved, it expanded the complexity of animal behaviour, including behaviours dependent on calcium. Gauberg’s study has also discovered differences between Trichoplax and human CaV2 channels that can reveal unique aspects of nervous system evolution.
One contrast between these animals is that TCaV2 is relatively insensitive to heavy metals and to neurotoxins compared to the human channels. This indicates that the CaV2 gene sequence has changed over time, making the human CaV2 channel more sensitive to toxins. The differences in TCaV2 and hCaV2 channel sequences can help us narrow down the regions of the channel bound by toxins or heavy metals.
Another fascinating contrast between the two channels relates to how CaV2 can be regulated. In humans, G proteins are a category of protein that interact with CaV2 channels to modulate how much calcium enters the cell. Gauberg found that human CaV2 is susceptible to regulation by Trichoplax G proteins. The surprising finding is that the Trichoplax G proteins cannot regulate their own TCaV2.
So it seems that the human CaV2 channels have gained the ability to be regulated by G proteins over evolutionary time. This may be because in the human brain the cell-cell signaling is very complex and CaV2 channels need more “fine tuning” compared to the Trichoplax CaV2 channel.
The multiple revelations of this outstanding study demonstrates that the more we study different animal nervous systems—from Trichoplax to snails to insects—the more we can learn about our own.