A recent paper from Dr Natalie Hoffmann and Prof Heather McFarlane has revealed unexpected discoveries into how the components of the plant cell wall are assembled. The plant cell wall makes natural fibres durable, makes wood strong, and provides energy in biofuels, all of which make the cell wall an important part of the world economy. The insights from their study will allow rational design to improve biological polymer synthesis without causing plant growth defects.
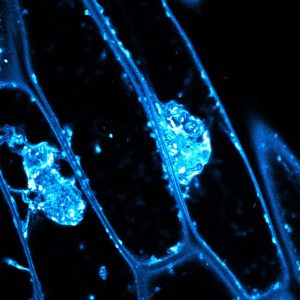 For 15 years, plant scientists have observed aberrant masses in specific cell wall mutants (as in the accompanying image). The reason for these aggregations wasn’t clear. They clumped together pectin, hemicelluloses, and glycoproteins, all components of the cell wall. Normally, these cell wall components are trafficked smoothly through the cell in vesicles that shuttle them to the surface for secretion.
For 15 years, plant scientists have observed aberrant masses in specific cell wall mutants (as in the accompanying image). The reason for these aggregations wasn’t clear. They clumped together pectin, hemicelluloses, and glycoproteins, all components of the cell wall. Normally, these cell wall components are trafficked smoothly through the cell in vesicles that shuttle them to the surface for secretion.
Plant scientists struggled to explain clumping in the mutants. The vesicles are moved on actin strands, so is an actin defect responsible for the buildup? Is one of the cell wall components causing aggregation in mutant plants? Is there a signal travelling through the mutant plants that is activating aggregation?
The answer came from Hoffmann and McFarlane’s precise and detailed studies. Hoffmann screened cell wall mutants for intracellular aggregations by exploiting the observation that aggregations are strongly labeled by FM4-64 dye.
Hoffmann’s diligent screening observed that mutants in the XyG enzyme MUR3 displayed severe aggregations. The defect was so severe that Hoffmann could see large subcellular organelles trapped in the aggregations under the electron microscope. XyG may act like a fastener that keeps the cellulose in the cell wall together. Plants entirely lacking XyG showed minimal defects and many XyG synthesis mutants were similarly unaffected.
MUR3 is a xyloglucan galactosyltransferase, which adds a galactose sidechain to the XyG molecule. Pursuing defects linked to this specific change, Hoffmann found multiple mutant lines with reduced XyG D-galactose sidechains that developed aggregations.
Normally, galactose molecules added to XyG make it soluble in the cell. So in this paper Hoffmann has shown that when a mutant xyloglucan does not have a sidechain attached to it, XyG polymers clump together, and other components get packaged with it on the way through the cell.
Hoffmann performed a wide variety of additional assays for this paper, a testament to her enormous technical abilities. She found that free organelles travel normally along actin, so actin defects are not responsible for aggregation. Inhibiting signals from cell wall damage or other forms of stress do not prevent aggregation, so defective signaling is not causing aggregation, either.
They concluded that sidechains on polysaccharides prevent precipitation and ensure efficient packaging and secretion to the plant cell wall. After secretion, polysaccharides can then be modified in the cell wall by cell wall-localized enzymes to fine-tune cell wall biophysical properties.
Together, Hoffmann & McFarlane’s work demonstrated that some components of the cell wall must be made in the correct format (such as the side chains on XyG) for them to be efficiently secreted. Therefore, future work to modify the plant cell wall should focus on changing cell wall polysaccharides after they have been made and secreted, for example by targeting cell wall localized polysaccharide modification enzymes.
This pivotal research is available in the journal Developmental Cell as “Xyloglucan sidechains enable polysaccharide secretion to the plant cell wall”
