The way embryonic cells stick to each other is given a new twist by studies of the sugar coating around developing tissues. The Winklbauer lab has shown that the glycocalyx sugar coat mediates cell-cell attachment across significantly large distances between cells. These surprising results are published in the journal PNAS as “Cell-cell contact landscapes in Xenopus gastrula tissues”.
Debanjan Barua began graduate studies in Prof Rudolf Winklbauer’s lab by studying Brachet’s cleft, a tissue-boundary in the frog embryo where the migrating mesoderm tissue travels adjacent to structured roof ectoderm. Most studies on cell-cell adhesion in the embryo focus on cadherin, which allows cells to sense each other over short distances. Scientists can clearly detect these interactions in monolayered tissues where individual cells are connected across relatively short distances.
However, in multilayered tissues undergoing rapid cell-cell rearrangement (as in Brachet’s Cleft tissues), cell-cell interactions seem amorphous. Barua worked to describe the landscape of these interactions and identify the adhesion components involved.
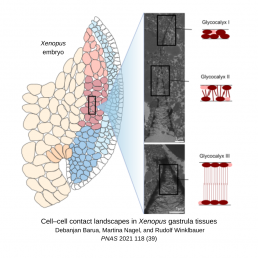
On the cell surface of embryonic tissue is a fibrous mesh called glycocalyx, which is composed of different sugar compounds. It is often described as a barrier to cell-cell adhesion in that the large size of its components hinder interactions between cells. It has a net negative charge, which Barua exploited by staining the cells with positively charged, electron-dense lanthanum to visualize the glycocalyx.
He was excited to observe the long, chain-like molecules of the glycocalyx interpenetrating across the gap between adjacent cells, contradicting the view of glycocalyx as a barrier. To explore the function of glycocalyx in cell-cell attachment, Barua assessed the geometrical angle and distance of gaps between cells to map the spectrum of interaction in normal embryos compared embryos where he disrupted gene expression.
Barua disrupted several transmembrane glycocalyx components such as syndecans, which are known to mediate adhesion with the extracellular matrix. In his study, syndecan depletion caused jagged gaps between cells, reducing cell-cell contacts to small points, but with less effect on cell density. This shows that syndecans play a role in cell-cell adhesion distinct from cadherins, which result in wide, long gaps when depleted. Moreover, his ground-breaking results showed that each component of the glycocalyx is unique in its modulation of cell-cell contacts in these tissues.
Having revealed a new element of embryonic development, Barua earned his PhD by virtually defending his thesis in 2021: “I could not have done it without Rudi [Winklbauer] showing me how to think about and approach my data”. Barua advises graduate students to take a break from collecting data to think about what their data means, as this tactic greatly benefited his studies. He is currently continuing his work in the Winklbauer lab by studying how ephrin signaling controls tissue separation at Brachet’s cleft and will begin his postdoctoral studies next year.