Dr Martina Nagel recently found the answer to a puzzle that she has been pursuing for 30 years in the laboratory of Prof Rudolf Winklbauer. She determined how the sheets of tissue that become the adult frog can migrate past each other while staying mostly separate as the embryo grows and develops into a tadpole. Using a suite of genetic tools she developed over 30 years and a novel X-ray imaging technique developed by Prof Ralf Hofmann of the Karlsruhe Institute of Technology, she could see that the cells in one of the sheets tiptoe over each other, rather than sliding on the surface of the other sheet that was considered the base layer.
This breakthrough was published in the journal Development as “Capillarity and active cell movement at mesendoderm translocation in the Xenopus gastrula”, with supporting contributions from co-authors Debanjan Barua and Dr Erich W Damm in CSB.
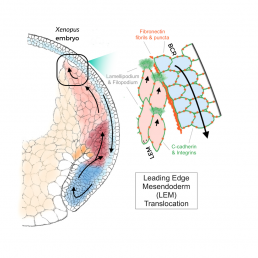
Before the Xenopus laevis embryo grows into a tadpole, a process known as gastrulation forms the layers of cells that become organs. As these cells fold over each other, the internal leading edge mesoderm (LEM) must move up under the downward-moving blastocoel roof (BCR).
Nagel is a leading expert in the signals that pass between these two sheets. She, Winklbauer and others have shown that the cues that guide cell movement reside on the BCR surface facing the LEM. Cadherin proteins mediate close cell–cell contacts, whereas adhesive cell contacts over intermediate distance occur through fibronectin (FN)-integrin interaction. The recognition of an FN-bound growth factor (PDGF-A) by its receptor (PDGFR-α) provides cell guidance: LEM cells adjacent to the BCR underlap each other with protrusions that are oriented in the direction of migration toward the pole of the embryo.
This shingle arrangement underlies the process of collective migration of the cells. The frog embryo is not transparent, and migration cannot be directly observed in the light microscope. Hofmann developed the imaging techniques exploited in the paper by using a third generation synchrotron beam. High energy emissions (30 keV) allow X-Ray tomography of opaque living embryos at the subcellular level, while also providing a wider field of view than conventional fMRI. Even though they can delicately tune the beam to keep the embryo alive, the team was limited to scanning embryos every 10 minutes, as more frequent images would cook the egg.
Collaborating with Hofmann, Nagel observed LEM cells detaching regularly from the external BCR base layer. Techniques that modulate the forms of PDGF and the levels of FN and cadherin demonstrated that these cells nevertheless depend on proximity to the BCR for guidance.
Nagel comments that “Rudi is always trying to bring his physics into my biology”, and indeed, Winklbauer calculated stress and pressure patterns due to active cell movement at the LEM wedge. These equations show that the shingle arrangement of cells in this liquid-like tissue produces a mechanical effect that ensures proximity of the LEM to the BCR without the need for direct, permanent contact except at the LEM tip. The tip cell-BCR contact mediates mechanical interactions that can be characterized in a force diagram that successfully modeled the observed movement, providing further support for Nagel’s conclusion.
Nagel’s very first paper demonstrated the shingle arrangement of the LEM using electron microscopy and cell culture. She is very happy that, after 30 years, her new paper finally shows the purpose of this arrangement in living embryos. Altogether, these results demonstrate that, to move across the BCR the shingled cells of the LEM crawl over each other instead of on the BCR itself, using an elaborate mechanism to hover over the BCR base layer and to collect directional information while only rarely touching the BCR.
Many thanks to Yunyun Huang of the Winklbauer lab for the accompanying explanatory diagram.