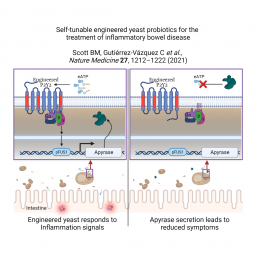
A new probiotic yeast, engineered at the University of Toronto and extensively tested at Bringham & Women’s Hospital (BWH) was designed using synthetic biology to sense and respond to inflammation, and has been shown to reduce IBD in mice. Inflammation can be a healthy response to infection that helps our bodies to recover, but chronic inflammation can cause long term problems like inflammatory bowel disease (IBD). Existing IBD treatments inhibit inflammation throughout the body regardless of where and when inflammation occurs, which can lead to significant side effects.
In developing this treatment, Benjamin Scott, a PhD student at CSB in the Chang and Peisajovich labs, noted reports of elevated levels of extracellular ATP (eATP) during inflammation and reasoned that detecting and responding to eATP could balance pro- and anti-inflammatory signals. He benefited from the expertise of Prof Chang, a leading expert in GPCR proteins. GPCRs function as sensors for a variety of molecules, including those that are present during inflammation in the intestine. GPCRs are used in engineered yeast cells to understand the ways genes are activated and more recently for the circuitry of synthetic biology.
Scott started his experiments by adding a human GPCR protein known as P2Y2 into yeast to detect eATP. He engineered the P2Y2 yeast to turn on a second synthetic component, a red fluorescent protein, if P2Y2 detected eATP. In his first tests, the crimson colour was only seen at extremely high levels of eATP. To improve human P2Y2’s sensitivity, protein variants were randomly generated and the reddest yeast revealed P2Y2 variants that could detect levels of eATP found in inflamed tissue.
With the ability to detect levels of eATP found in the body, Scott could now turn to ways of reducing the levels of eATP around the yeast cells. An enzyme called apyrase breaks down eATP, so Scott swapped out the red fluorescent protein for potato apyrase, the most active apyrase known. The resulting yeast strains were successful at both detecting and reducing pro-inflammatory levels of eATP in a test tube.
Demonstrating reduced intestinal inflammation still required testing in an animal model. Peisajovich and his long-time friend Prof Francisco Quintana (BWH) were developing the idea of a “living drug”, engineered cells that produce therapeutic effects. Work moved to Quintana’s lab, which studies autoimmune diseases using mice. Dr. Cristina Gutiérrez-Vázquez and others in the Quintana lab undertook the treatment of lab mice with the engineered yeast. She demonstrated that there were no negative effects on normal intestine, and that the engineered yeast was cleared out of the mouse’s gut within 24 hours.
Her detailed analysis of intestinal inflammation demonstrated the ability of the engineered yeast to reduce inflammation, a key step in alleviating inflammatory bowel diseases. This was a huge result that the labs celebrated, and they filed a patent for their demonstrated probiotic based on these results.
Their work is published in Nature Medicine as “Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease”.