Preparing to Push: Epigenetic analysis of uterine muscle cells reveals molecular basis of term labour
Answers to some questions in science require the development of new technologies; molecular biologists once examined selected pieces of DNA and the proteins that bound them, but advances in computation and instrumentation mean biologists can now determine where proteins bind across the whole genome, and look at expression of all the genes in a cell in one experiment.
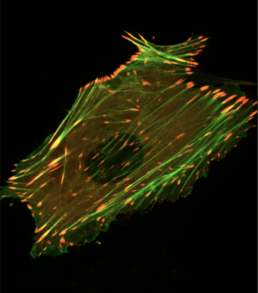
Professor Jennifer Mitchell of U of T's Department of Cell & Systems Biology is now revisiting her PhD work on how the transition from pregnancy to labour occurs by using these new techniques. During pregnancy the uterus is not able to contract synchronously, but just before labour the muscle layer of the uterus changes so that it can produce the coordinated contractions required for birth. Mitchell’s PhD work studied control of a “gap-junction” protein that allows cell-to-cell communication. She examined a class of proteins designated AP-1 that regulate the amount of gap junction alpha 1 (GJA1) protein in the myometrium, the muscle layer of the uterus.
During labour, cells in the myometrium transition from a dormant to a contractile (pushing) state. Expression of the Gja1 gene is associated with this transition, and Mitchell identified AP-1 proteins that could cause the expression of Gja1 just before the onset of labour. Although she determined certain AP-1 proteins could turn on Gja1, the technology of the time couldn’t evaluate the role of these proteins on a genome-wide scale.
In collaboration with Temerty Faculty of Medicine Professor Oksana Shynlova of the Lunenfeld-Tanenbaum Institute, Mitchell and her two graduate students, Virlana Shchuka and Luís Abatti, developed high-throughput techniques to analyze protein binding to DNA and assess RNA expression across the whole genome at different phases of pregnancy. They examined not just Gja1, but all the genes turned on or off at multiple time points during gestation in mice.
By analyzing the proteins associated with DNA around these genes, Mitchell’s team identified epigenetic modifications in mouse myometrium. Epigenetic modifications change the way the DNA is packaged at specific regions, and Mitchell’s observed modifications permit binding of AP-1 proteins. The results of this research have been published in the peer-reviewed journal PLOS Biology: “The pregnant myometrium is epigenetically activated at contractility-driving gene loci prior to the onset of labor in mice”.
One remaining mystery is that the observed epigenetic changes occur four days prior to the onset of labour, so there must be a further signal that turns on contractile genes. It is likely that premature gene expression leading to early myometrial contractions and preterm labor in humans is the result of this signal occurring too early. Mitchell and Shynlova’s teams have recently received funding from the Canadian Institutes for Health Research to delve deeper into this mystery with the goal of identifying genes and protein targets for drugs to prevent preterm labor in humans.
Innovative problem solving leads to 2020 Dean’s Outstanding Technical Service Award for Lisa Matchett
Congratulations to Lisa Matchett for receiving the 2020 Dean’s Outstanding Technical Service Award. The award recognizes staff members whose excellent contribution to technical services has improved teaching and research.
Using innovative problem solving as a Teaching Lab Technician, Lisa has improved the efficiency of the teaching labs and enhanced student learning in courses such as first-year Cell and Molecular Biology. Lisa also strives to maintain a safe working environment for students and researchers at Ramsay Wright, as a Worker Co-Chair of the Health and Safety Committee.
Vice-Dean Vincent Tropepe expressed his appreciation to Lisa on the “excellent service that you provide to CSB and the faculty.”
Congratulations Lisa!
Cell biology meets engineering in collaborative XSeed grant for Professor Sergey Plotnikov to study wound closure in fruit flies
CSB Professor Sergey Plotnikov has been awarded XSeed funding to identify protein targets that can accelerate wound healing. The XSeed program catalyzes cross-disciplinary partnerships between investigators from the Faculty of Applied Science and Engineering and other faculties.
The grant was awarded in collaboration with Professor Fernandez-Gonzalez from the Institute of Biomaterials and Biomedical Engineering. Plotnikov and Fernandez-Gonzalez will leverage their expertise in wound repair and cell mechanics to probe the mechanisms of cell movement to the site of wounding.
Animal cells are embedded in an extracellular matrix (ECM) and protein interactions through the ECM allow cells to maintain their shape and structure. Cells adhere and migrate through the ECM using multi-protein structures called focal adhesions. Plotnikov and Fernandez-Gonzalez will examine the dynamics of cell-ECM adhesions after wounding.
They will investigate how talin, a core protein in focal adhesions, allows cells to move to the site of an injury, thereby closing the wound. Wound closure in talin mutants with either weaker or stronger ECM binding will be compared to normal talin. Wounds will be induced through laser nanosurgery, and the accumulation of talin and the assembly of focal adhesions at the injured site will be recorded using cutting-edge spinning disc confocal microscopy. The results of this study will establish whether talin-based cell-ECM adhesions that allow cells to move are required for wound healing.
Cell movement in response to wounding is regulated using transient receptor protein (TRP) ion channels. These channels respond to mechanical stresses exerted on cells by altering cellular calcium ion levels. TRP-mediated calcium signaling controls cell-ECM adhesions to regulate cell movement. Plotnikov and Fernandez-Gonzalez will suppress different components of the calcium signaling pathway to investigate how calcium signaling controls the assembly of focal adhesions and the movement of cells during wound closure.
Adult wounds heal much slower than embryonic wounds and often result in scarring and infection. By studying cell-ECM adhesions and wound closure in fruit fly embryos, Plotnikov and Fernandez-Gonzalez will be able to identify components of the protein pathway that can be modified with the goal of accelerating wound healing in adults.
Parasitic plant study by Professor Shelley Lumba reveals unexpected pathway to germination of witchweed seeds
The crop fields of sub-Saharan Africa have fallen under a spell cast by the witchweed Striga hermonthica. Every year, this parasitic plant targets and destroys over eight billion dollars worth of staple crops, leading the UN to declare Striga infestations as a major impediment to poverty alleviation in Africa. A new discovery from researchers at U of T could provide tools to prevent Striga seeds from germinating in farmers’ fields.
Striga’s success comes from its ability to lie dormant in the soil for years, lying in wait for a crop host to infect. When a host plant begins to grow, it emits a small molecule called strigolactone into the soil. This chemical acts as a cue for Striga to germinate, telling the parasite that a host is nearby.
Due to its parasitic nature, studying Striga in the laboratory is challenging. Plant scientists use the model plant Arabidopsis thaliana to study germination but unlike Striga, Arabidopsis does not germinate in response to strigolactone. Professor Shelley Lumba’s lab in the Department of Cell & Systems Biology (CSB) decided to learn more about the action of strigolactone on germination by expressing Striga’s strigolactone receptor protein in Arabidopsis.
As the results came in, excited discussions arose in the hallways of CSB and at the coffee stand, provoked by the surprising revelation that strigolactone could trigger germination independent of Arabidopsis’ dominant germination pathway.
Normally Arabidopsis seeds (and crop seeds) don’t germinate in storage because of the activity of a group of proteins called DELLA domain repressors. When these seeds sense enough water and warmth, their cells produce a hormone known as gibberellin that enables breakdown of DELLA proteins, kickstarting the process of germination. Lumba found that activated Striga receptors in Arabidopsis circumvented gibberellin to cause germination.
The signal that bypasses gibberellin takes an unexpected path from the strigolactone receptor through the cellular KAI2 protein, which had no previously known receptor partner. The KAI2 signaling pathway inactivates the SMAX1 protein to allow germination even in the presence of DELLA repressors. These details, revealing how Striga strigolactone receptors act as the dominant signaling component in parasitic plant germination, will refine the methods used to prevent the growth of these devastating weeds.
The approach used by the Lumba lab shows that receptors for known hormones can be adopted to analyze orphan receptors, which may have broader utility in understanding hormone signaling in both plants and animals. These results were published in a recent Nature Plants article: “SMAX1-dependent seed germination bypasses GA signalling in Arabidopsis and Striga“.