 Some proteins can detect light. Others are sensitive to temperature. Some act as taste receptors. Opsin proteins can do it all.
Some proteins can detect light. Others are sensitive to temperature. Some act as taste receptors. Opsin proteins can do it all.
Professor Belinda Chang and colleagues have received $2 million in funding to demystify how a single class of protein can execute and retain such a broad variety of sensory functions, given that evolutionary pressures might easily compromise one in favour of another.
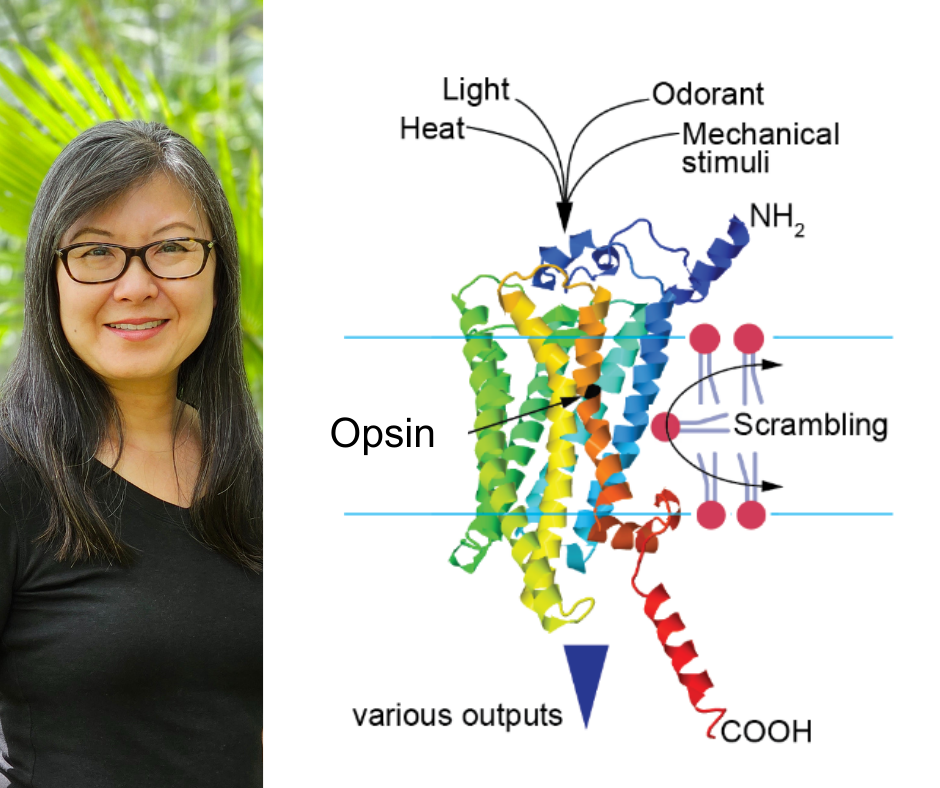
Opsins are known as a visual pigment essential for vision in every species with eyes, with rhodopsin Rh1 being the most studied. But Chang’s colleagues have shown in select species that opsin moonlights outside of eyesight to take on roles in sensing heat, touch and taste.
Rh1’s versatility extends beyond the senses, with the finding that Rh1 can act at cell membranes to scramble proteins inside-out. This defies the view that proteins are tailored to do one job, and shows that evolutionary pressures can adapt proteins to unexpected roles.
With funding from the Human Frontiers Science Program, the researchers will target the Rh1 opsin from fruit flies to understand how sequence variation in nature is limited by multiple functions, which regions of the protein are involved, and how the protein maintains these functions in the face of conflicting evolutionary pressures.
The first step is to identify the mutations that Rh1 can tolerate without interfering with its ability to fold and activate. The Chang lab has a yeast-based assay that puts Rh1 on the surface of the cell to be activated by light. When Rh1 is properly folded and activated by light, the yeast glows green.
This system allows the researchers to assess the abundance, localization and signaling of Rh1 in a high-throughput manner. Exhaustive coverage of Rh1mutants with all possible amino acid substitutions will reveal every variant which can still properly fold and respond to light.
A key question is why some of these mutants, despite folding properly, are not found in nature. “We think that a substantial portion of those might not exist in nature because of the other functions taken on by rhodopsin,” says Chang. “These other functions may limit the amount of sequence variation Rh1 can tolerate and still respond to light.”
The next step is to synthesize the mutants that should theoretically exist but that are not found in nature. These mutants will be tested in flies to determine if the flies can gain extraordinary abilities in taste, thermal reception, touch, or other activities.
The collaborative nature of this project will ensure a multifaceted view of the evolutionary constraints on the Rh1 protein. Congratulations, Professor Chang!
